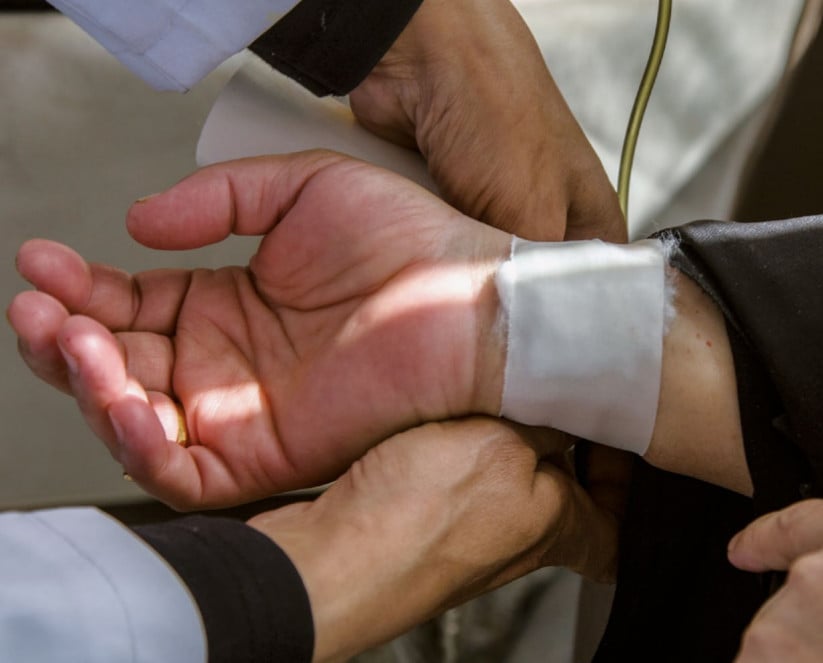
Roche intimidates clinical drug trial participant following report
Following the publication of a critical report by SOMO on clinical drug trials in Egypt, pharmaceutical company Roche made reprehensible efforts to have damaging information removed from the report. They placed one of the interviewed Egyptian clinical drug trial participants under pressure to withdraw her story and brought a lawsuit in Switzerland against one of the report’s authors.
Industry-sponsored clinical drug trials in Egypt
On 21 June 2016, Wemos, SOMO, Public Eye, Egyptian Initiative for Personal Rights and Shamseya jointly published an investigation into the use of vulnerable clinical drug trial subjects in Egypt. The report, entitled ‘Industry-sponsored clinical drug trials in Egypt: Ethical questions in a challenging context’ showed how international ethical standards are violated. Pharmaceutical companies have failed to observe Egyptian law (for as far as it exists) as well as their own codes of conduct.
The Dania case
The report describes the case of 65-year-old cancer patient Dania. Just as half of the Egyptian population, she has no health insurance. Her first cancer treatment was paid for by the state, but after two years, the cancer returned. Second requests for state-sponsored treatment are often denied. But Dania was told that she could take part in clinical trials for a new drug. The side effects were serious: the drugs caused severe diarrhoea, incontinence, loss of her nails and skin burns. She also had to pay for two operations for cataracts herself, as the side effects were not acknowledged to be the result of the trial.
Link to Dutch newspaper article on the case(opens in new window)
The court case
Five months after the report was published (which had already been sent to all the companies involved for verification), a Swiss delegation from Roche appeared at Dania’s door. Under pressure, she authorised a Swiss solicitor, who was connected to Roche in a number of ways, to start a legal procedure in her name. She also signed a statement in which she denied the side effects. The Swiss court, however, found her objection to the publication implausible and in February (2017) dismissed her complaint on all counts.
Do you need more information?
-

Irene Schipper
Senior Researcher
Partners
Related news
-
 EU health data law rolls out the red carpet for Big TechPosted in category:Long read
EU health data law rolls out the red carpet for Big TechPosted in category:Long read Irene SchipperPublished on:
Irene SchipperPublished on: -
Civil society coalition urges EU to put the interests of patients and citizens at the heart of the European Health Data SpacePosted in category:Published on:Statement
-



