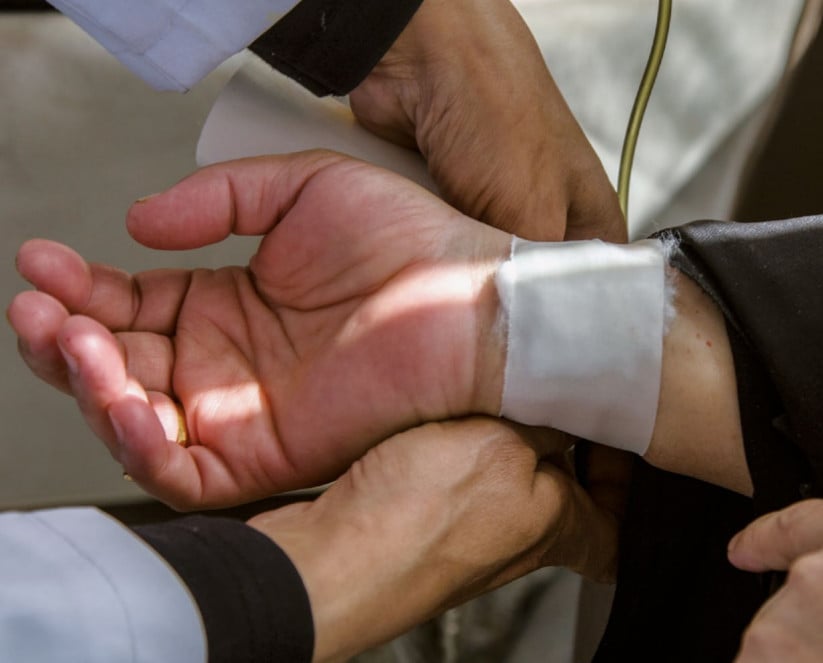
Industry-sponsored clinical drug trials in Egypt
Ethical questions in a challenging context

When engaging in clinical trials in low- and middle-income countries with limited access to treatment, pharmaceutical companies should ascertain that the safety and rights of participants are properly protected and that their practice are in line with the highest ethical standards. They should also make sure that the medications tested in those countries are available at an affordable price. This study brings new evidence that unethical practices occur in TNC sponsored clinical trials conducted in Egypt, and that drugs tested in Egypt are not systematically available to and mostly unaffordable for the Egyptian population.
Do you need more information?
-

Irene Schipper
Senior Researcher
Partners
Publication

Related news
-
 EU health data law rolls out the red carpet for Big TechPosted in category:Long read
EU health data law rolls out the red carpet for Big TechPosted in category:Long read Irene SchipperPublished on:
Irene SchipperPublished on: -
Civil society coalition urges EU to put the interests of patients and citizens at the heart of the European Health Data SpacePosted in category:Published on:Statement
-



